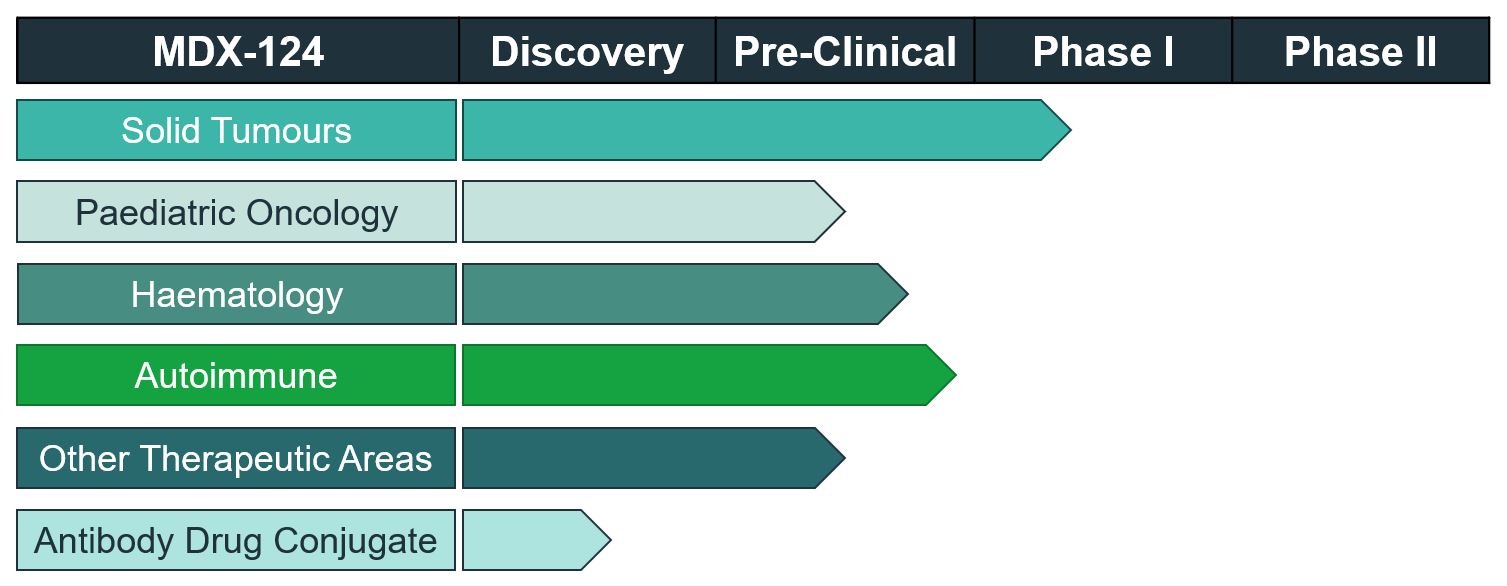
The ongoing Phase Ib ‘ATTAINMENT’ study is a modular, multi-arm, first-in-human study to evaluate the safety and tolerability of MDX-124, alone and in combination with anti-cancer treatments, in participants with locally advanced, unresectable or metastatic solid malignancies.
The study will establish the Recommended Phase II Dose via rapid dose escalation. The efficacy of MDX-124 will then be evaluated in front-line therapy, in combination with standard-of-care cancer treatments.
The Chief Investigator is Professor Daniel Palmer of the Liverpool Clinical Trial Centre. Patients are also being enrolled by Professor Sarah Blagden at the Early Phase Trials Unit, University of Oxford and Dr. Stefan Symeonides at the Edinburgh Cancer Research Centre.
Find out more about the ATTAINMENT study via Cancer Research UK or the ISRCTN Registry.
MDX-124 is a humanised monoclonal antibody, and the first agent to specifically target and inhibit annexin-A1, a protein known to play a key role in the development of several cancers and autoimmune diseases. In pre-clinical studies it has shown significant anti-cancer activity as a single agent as well as powerful synergy with standard-of-care therapies. It was recently granted EMA Orphan Designation and an MHRA Innovation Passport for the treatment of pancreatic cancer.
MDX-124 has also shown considerable potential for the treatment of various autoimmune diseases, and Medannex is planning further clinical trials in this area.